Adjustable Gravitational Valve for the Treatment of Hydrocephalus
Take the Headaches Out of Treating Hydrocephalus with Gravitational Technology That Protects from Overdrainage
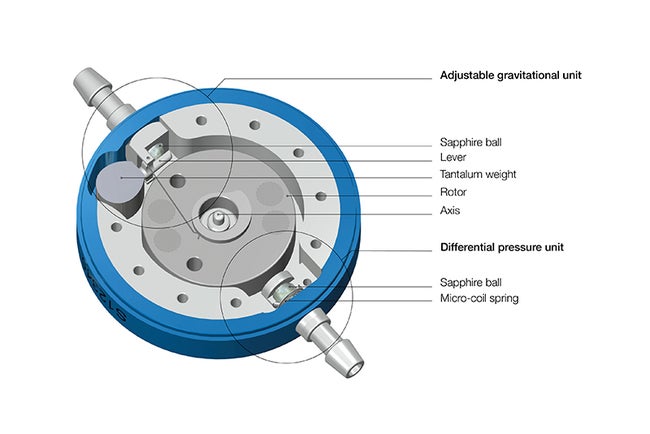
The M.blue Valve is a position-dependent valve designed for hydrocephalus treatment. It combines a fixed differential pressure unit with an adjustable gravitational valve, creating an all-in-one system that automatically adjusts based on the patient’s positions. This gravitational technology for hydrocephalus management helps counter the risks of possible overdrainage complications.
The only adjustable gravitational valves
M.blue Valve - The 2-in-1 technology features an adjustable 0-40 cmH2O1 gravitational unit combined with a fixed differential pressure unit. This combination adapts to a patient’s mobility, growth, and changes in condition. Differential pressure units are available in 0, 5, 10 and 15 cmH2O.
M.blue plus Valve- Programmable differential pressure valve 0-20 cmH20 combined with a programmable gravitational valve 0-40 cmH20.
Jamie Wright's hydrocephalus journey with M.blue Valve System
Jamie Wright's life was transformed by the M.blue Valve System. Diagnosed with hydrocephalus, she faced challenges but never gave up. Discover how the M.blue and M.blue plus Shunt System helped improve her health, enabling her to help others as a physician.
M.blue and M.blue plus Valve features:
- Unique pressure adaption to optimize individual patient needs.
- Protection against overdrainage prevention through individually and continuously adjustable combined opening pressure from 0-60 cmH2O.1
- MRI Conditional hydrocephalus valve up to 3 Tesla – No need for X-ray verifications post-MRI, minimizing patient radiation exposure.2
- Safe from unintentional adjustment from everyday magnets.3
- Designed to be intuitive, secure and comfortable to adjust.
- Precision engineering.
- Low profile.
- Robust and durable: made of titanium.
Better protection. Better outcomes.
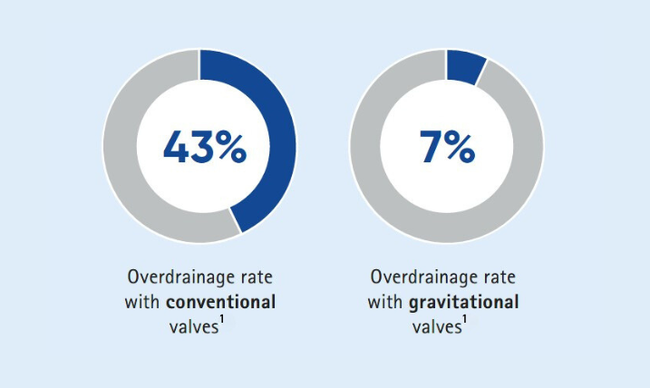
Clinical studies have shown that MIETHKE gravitational technology for hydrocephalus management reduces the risk of revisions and overdrainage complications.1,4-6
- Valve survival rates up to 90% at 12 months.5
- Implanting a gravitational valve prevents one additional overdrainage complication in about every third patient.1
- Improved patient outcomes, including minimizing overdrainage complications and improved cognitive function, with gravitational valves compared to conventional ones.7
Simplified valve adjustment and verification
M.blue plus Instruments allow users to measure, verify and adjust the pressure level on the M.blue Valve’s adjustable gravitational unit (0-40 cmH2O) as well as the pressure level on the adjustable differential pressure unit (proGAV® 2.0 Valve) of the M.blue plus Valve. The instruments help to offer simple steps for the physician and make the adjustment process comfortable for patients.

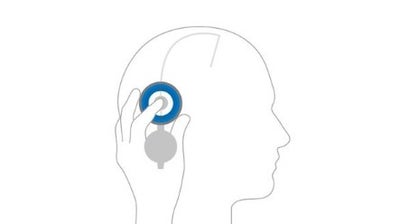
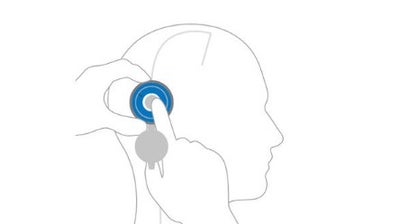
Locate
Locate the valve by palpating the area with your finger through the open M.blue plus Compass.
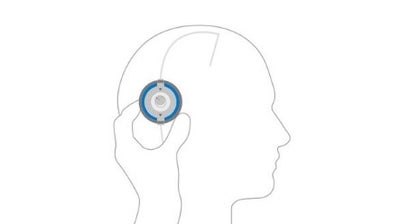
Verify
Close the M.blue plus Compass and use the floater to lock location and read the current valve opening pressure setting.
Adjust
With the help of the inserted adjustment ring, the valve opening pressure can easily be set to the desired level. After setting the valve opening pressure, it is advisable to double-check the pressure level settings.
Contact us
Please complete the form below and an Aesculap representative will contact you.
Contact us
Please complete the form below and an Aesculap representative will contact you.
Your request could not be submitted. Please try again.
warningReferences:
1Lemcke J, Meier U, Muller C, et al. Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: a pragmatic, randomised, open label, multicentre trial SVASONA. J Neurol Neurosurg Psychiatry 2013;848:850-7
2Aesculap, Inc. Data on File
3Spader HS, Ratanaprasatporn L, Morrison JF, Grossberg JA, Cosgrove GR. Programmable shunts and headphones: Are they safe together? Journal of Neurosurgery: Pediatrics. 2015;16(4):402-405. doi:https://doi.org/10.3171/2015.1.peds14400
4Golz L, Lemcke J, Meier U. Indications for valve-pressure adjustments of gravitational assisted valves in patients with idiopathic normal pressure hydrocephalus. Surg Neurol Int 2013;4:140.
5Sprung C, Schlosser HG, Lemcke J, et al. The adjustable proGAV shunt: a prospective safety and reliability multicenter study. Neurosurgery 2010;663:465-74.
6Thomale UW, Gebert AF, Haberl H, et al. Shunt survival rates by using the adjustable differential pressure valve combined with a gravitational unit proGAV in pediatric neurosurgery. Childs Nerv Syst 2013;293:425-31.
7Suchorska B, Kunz M, Schniepp R, et al. Optimized surgical treatment for normal pressure hydrocephalus: comparison between gravitational and differential pressure valves. Acta Neurochirurgica. 2015;157(4):703-709. doi:https://doi.org/10.1007/s00701-015-2345-4
For MRI safety information, please see Instructions for Use.
